April 4, 2016 – On this Monday we conducted an experiment which aimed to determine the specific heat of brass,copper and aluminum using a calorimeter.
The amount of heat required to change the temperature of an object depends on its specific heat capacity c, its mass m, and the change in temperature ΔT:

The specific heat capacity is an intrinsic property of an object. It depends on the material that the object is made of. The specific heat capacity is 4.184 J/(g C°) for water.
So what is a calorimeter anyway? A calorimeter is composed of an insulated vessel, which prevents heat released by a thermal interaction inside the vessel to escape into the surroundings.
In this activity, the calorimeter consists of stacked styrofoam cups. The calorimeter was partially filled with tap water. The sample was then mixed into the tap water and the ΔT of the tap water was measured. If the calorimeter initially had the same temperature as the tap water, the amount of heat lost by the hot water is equal to heat absorbed by the tap water and the calorimeter:

The experiment was divided into two parts. In the first part, the heat capacity of the calorimeter was to be determined. The amount of heat gained by the calorimeter can be expressed as
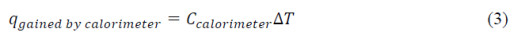
where Ccal is the heat capacity of the calorimeter. By monitoring the temperature change of tap water in the calorimeter when hot water is added, Ccal was determined:
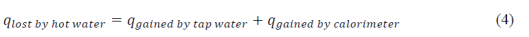
By using equations (1) and (3), the specific heat of the calorimeter can be determined by measuring the temperature changes in the hot water and tap water. To get the temperature of the mixture of hot and tap water upon mixing, the temperature of the water mixture was tracked over time in 20 second intervals for 5 minutes. Table 1 below shows the data obtained from one trial.
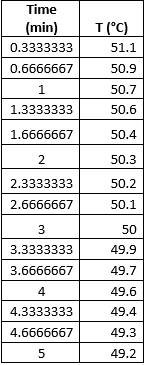
Table 1. Temperature measured over 20 second-intervals.
The logarithm of the temperatures vs the time was plotted to obtain the final temperature of the mixture from the exponential of the y-intercept of the graph. Figure 1 below shows the graph of one of the trials.
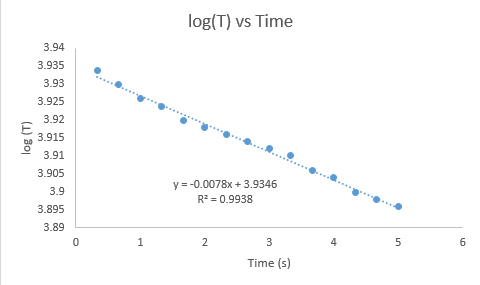
Figure 1. Logarithm of Temperature vs Time
Using equation (1), Qlostbyhotwater and Qgainedbytapwater was computed. Using Equation (4), Qgainedbycalorimeter was computed. Ccal was then computed using equation (3). Three trials were done to obtain the best value for Ccal by getting the average. The obtained average Ccal was 95.51.
The second part of the experiment was to determine the specific heat of copper,aluminum ,and brass. The metal sample was placed in boiling water, raising its temperature to approximately 100 °C. Spontaneous heat transfer happens between the metal, water and the calorimeter when the hot metal is placed on the calorimeter containing room-temperature water :
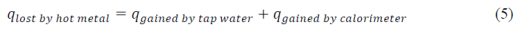
The same procedure in the first part was done to obtain the specific heat of the metals. The obtained specific heats of the metals were 0.379, 0.424, and 1.26 for brass, copper, and aluminum, respectively. The percent errors of the obtained specific heats were 0.26%, 9.8%, and 28.6% for brass,copper, and aluminum, respectively. The errors can be attributed to the loss of heat as the metal was transferred from the boiling water to the styrofoam.
The experiment was light. It wasn’t that stressful. I hope experiments are always like this. Hehe 🙂
